

All unsere Health IT-Lösungen
Leistungsstarke Health-IT für Krankenhaus und Radiologie

ORBIS U - die nächste Generation unseres KIS
Neue Architektur, mehr Komfort, stärkere Vernetzung


Mrd. Labor-Ergebnisse pro Jahr
Mio. Patienten-Akten aus +6100 Kliniken
Mio. Stationäre Aufnahmen pro Jahr
Mio. Notfall-Aufnahmen pro Jahr
Mio. Patienten pro Jahr
Unser Ansatz. Vom episodenzentrierten Denken zum Continuum of Care.
Die Integration von Diensten über das Continuum of Care wird durch eine dedizierte Plattform ermöglicht, die volle semantische Interoperabilität auf Basis offener Standards bietet, und die Modernisierung bestehender Anwendungen und die Schaffung eines offenen Ökosystems von Lösungen ermöglicht.

Die Prävention bewegt sich in drei Richtungen: Gesetzgebung, Befähigung der Patienten und Immunisierung, wobei der Patient und die Gemeinschaft eine große Rolle spielen.

Die klinische Erfahrung zeigt, dass Pathologien wie die Onkologie und die Kardiologie eine frühzeitige Diagnose und Behandlung erfordern. Im Continuum of Care ist es von grundlegender Bedeutung, flexible Lösungen einzusetzen, die Kliniker bei der Früherkennung unterstützen.

Schnelle, prädiktive und effiziente Tests. Große, konsolidierte Labore. Die Beschleunigung der digitalen Pathologie. Um Effizienz und Sicherheit zu gewährleisten, brauchen all diese Prozesse ganzheitliche Lösungen, Vernetzung von Projekten, Erfahrung, Kompetenz.
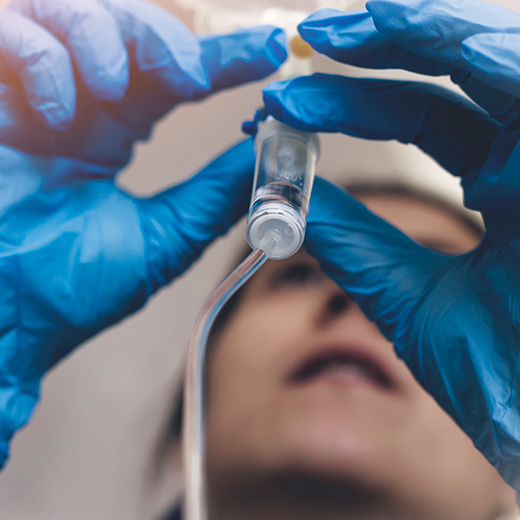
Im Ablauf der Patientenbehandlung benötigen alle Beteiligten Komponenten, die den Austausch von Informationen, klinische und pflegerische Bewertungen, das Management des Lebenszyklus von Medikamenten und die Integration mit Roboter- oder Gerätetechnologie unterstützen.

Die Rehabilitation wächst mit der Lebenserwartung und den damit verbundenen chronischen Krankheiten und Behinderungen. Das Continuum of Care benötigt Lösungen für die richtige Planung, Bereitstellung und Steuerung von Aktivitäten und Ressourcen.

Die Aktivitäten, die die Pflege nach der Behandlung und nach dem Krankenhausaufenthalt definieren, erfordert, dass der Patient intensiv einbezogen und zeitnah über seinen Behandlungsplan informiert wird und dass er bei der Anwendung des Plans auch in der häuslichen Umgebung unterstützt wird.

Der Patient, seine Familie und der Arzt werden von einem multidisziplinären Team von Spezialisten unterstützt, das klinische, pflegerische, psychologische und soziale Daten austauschen kann, sowohl im Krankenhaus, im Pflegeheim als auch direkt zu Hause.

